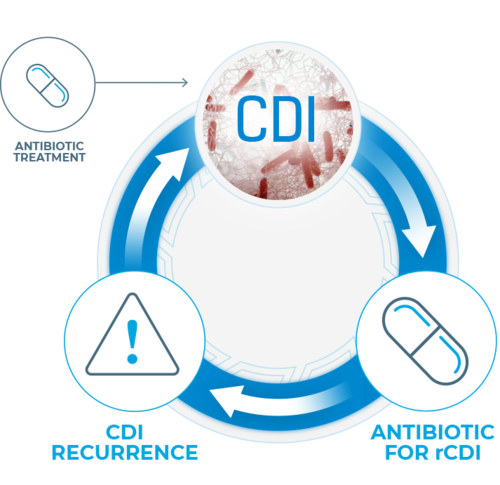
Up to 35% of patients will experience recurrence within 8 weeks after initial C. diff infection (CDI) diagnosis.1,2 A predominant risk factor for recurrence, antibiotic use has been shown to disrupt the ecology of the human microbiome, does not address the underlying dysbiosis, and is associated with increased risk of deadly infections such as recurrent C. diff.3-6
Longer exposure to multiple antibiotics and treatment with multiple antibiotics may increase the risk.7
Disruption of microbiota increases the risk of C. diff infection by providing a niche for the infection to flourish. Should the intestinal microbiota be disrupted by antibiotics, the effects may be long-lasting and the risk of C. diff infection may increase during continued therapy. Longer exposure to multiple antibiotics and treatment with multiple antibiotics may increase the risk.7-11
Thus starts a cycle of C. diff infection and reinfection—impeding microbiome recovery, exacerbating morbidity, and creating a substantial economic burden.1.7-14

Restoring a healthy gut microbiome is increasingly accepted as a promising treatment option for recurrent C. diff infection.9
C. diff infection can be more
dangerous when it recurs.
Can the power of the microbiome help
change the course of treatment?
Share this with colleagues!

References
- Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372(9):825-834.
- Cornely OA, Miller MA, Louie TJ, Crook DW, Gorbach SL. Treatment of first recurrence of Clostridium difficile infection: fidaxomicin versus vancomycin. Clin Infect Dis. 2012;55(suppl 2):s154-s161.
- DePestel DD, Aronoff DM. Epidemiology of Clostridium difficile infection. J Pharm Pract. 2013;26(5):464-475.
- Knight CL, Surawicz CM. Clostridium difficile infection. Med Clin North Am. 2013;97(4):523–536.
- Aukes L, Fireman B, Lewis E, et al. A risk score to predict Clostridioides difficile infection. Open Forum Infect Dis. 2021;8(3):ofab052.
- Langdon A, Crook N, Dantas G. The effects of antibiotics on the microbiome throughout development and alternative approaches for therapeutic modulation. Genome Med. 2016;8(1):39.
- Nelson WW, Scott TA, Boules M, et al. Health care utilization and costs of recurrent Clostrdioides difficile infection in the elderly: a real-world claim analysis. J Manag Care Pharm. 2021;27(7):828-838.
- Riddle DJ, Dubberke ER. Clostridium difficile infection in the intensive care unit. Infect Dis Clin North Am. 2009;23(3):727-743.
- Kelly CP. Can we identify patients at high risk of recurrent Clostridium difficile infection? Clin Microbiol Infect. 2012;18(suppl 6):21-27.
- Leong C, Zelenitsky S. Treatment strategies for recurrent Clostridium difficile infection. Can J Hosp Pharm. 2013;66(6):361-368.
- Smits WK, Lyras D, Lacy DB, Wilcox MH, Kuiiper EJ. Clostridium difficile infection. Nat Rev Dis Primers. 2016;2:16020.
- Van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368(5):407-415.
- Guh AY, Mu LG, Winston LG, et al. Trends in U.S. burden of Clostridioides difficile infection and outcomes. N Engl J Med. 2020;382(14):1320-1330.
- Feuerstadt P, Stong L, Dahdal DN, Sacks N, Lang K, Nelson WW. Healthcare resource utilization and direct medical costs associated with index and recurrent Clostridioides difficile infection: a real-world data analysis. J Med Econ. 2020;23(6):603-609.